Beyond-Use Dating and Labeling in Compounded Sterile Products
BUD means start by, not finish by. To avoid wasted doses and delays, label CSPs with clear language like “Start infusion by [date/time],” aligning with USP <797> and supporting safe, efficient nursing care.
Definition of Beyond-Use Date (BUD)
A Beyond-Use Date (BUD) is the date and time after which a compounded sterile product (CSP) cannot be used. According to USP <797> Pharmaceutical Compounding—Sterile Preparations, the BUD is determined based on microbial risk level, chemical stability, and sterility assurance. Unlike a manufacturer’s expiration date, which reflects the period a product maintains potency in unopened, controlled conditions, the BUD is assigned by the compounding pharmacy to ensure both safety (sterility) and stability (potency) once a preparation leaves the compounding environment.
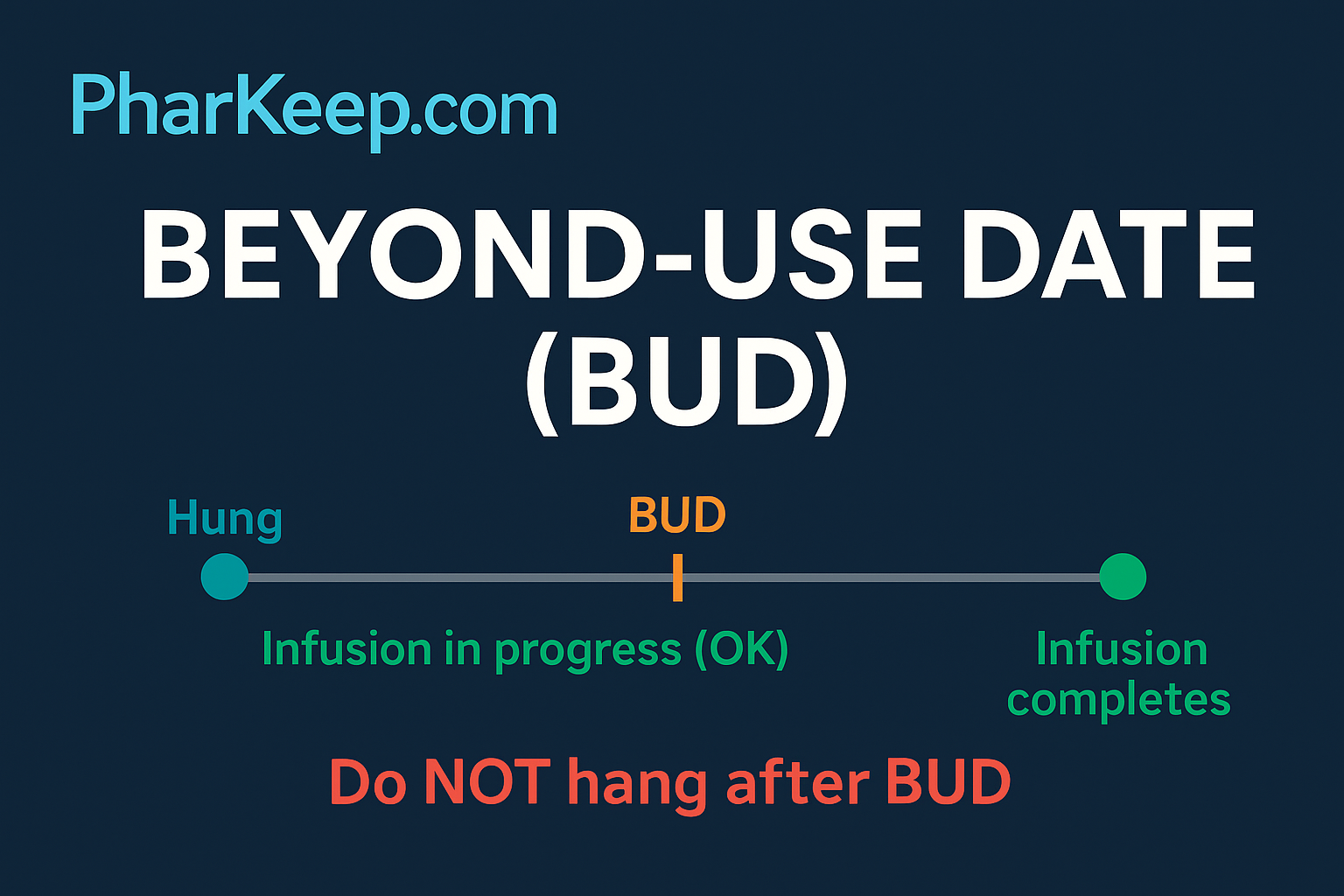
USP <797> intends for the BUD to indicate the last point in time that a CSP can be initiated, not the time it must be completely infused. Once the infusion has begun within the assigned BUD, the product remains valid for administration throughout the infusion period, provided it is otherwise stable and properly handled.
Common Misinterpretations by Nursing Staff
A frequent source of confusion arises in the clinical setting when nurses equate the BUD with the completion time of an infusion. For example, if a CSP has a BUD of 1400, some staff may incorrectly believe the infusion must finish by 1400, when in reality the requirement is only that the product be hung and started before 1400. This misinterpretation can lead to unnecessary discards of viable product, medication delays, or disputes between pharmacy and nursing staff.
Analysis of Labeling Phrasing
Several variations of BUD labeling have been used in practice:
- “Do not use after [date/time]”
- Issue: Ambiguous; may be read as “infusion must be completed before this time.”
- “Infuse completely by [date/time]” or “Start and complete infusion by [date/time]”
- Issue: Incorrect per USP <797>, since products can finish infusing after the BUD if started on time.
- “Do not hang after [date/time]”
- Strength: Direct and action-oriented.
- Issue: Ambiguous; may be read as “infusion product must be taken down after this time.”
- “Start Administration By [date/time]”
- Strength: Clearer and aligned with the intent of USP <797>.
Recommended Best Practice for Labeling
To reduce confusion and promote safe, compliant practice, the preferred verbiage for BUD labeling is:
➡ “Start infusion by [date/time]”
This phrasing:
- Emphasizes the required action (start/hang the infusion).
- Removes ambiguity around completion time.
- Mirrors USP <797>’s intent that the BUD refers to initiation of use.
An acceptable alternative is:
- “Must be hung by [date/time]”
Both phrases are directive, intuitive, and prevent the common misinterpretation that infusion must be completed by the BUD.
Clinical Implications and Teaching Point
Clear BUD labeling is a medication safety strategy. By selecting terminology that conveys “initiation of infusion,” pharmacists prevent unnecessary product waste and avoid delays in therapy. This reinforces the critical distinction between BUD (start time) and expiration date (end of shelf-life), and highlights how communication across interprofessional teams directly impacts patient care.
