Briumvi (Ublituximab-xiiy) Drug Information
Briumvi (Ublituximab-xiiy)
Mechanism of Action & Indications
Briumvi is a glycoengineered anti-CD20 monoclonal antibody that selectively depletes CD20-positive B cells, reducing relapse rates in relapsing forms of MS (RRMS, active SPMS, clinically isolated syndrome). Its enhanced antibody-dependent cellular cytotoxicity (ADCC) provides a 25–30x increase in B-cell depletion efficacy compared to earlier anti-CD20 therapies.
Dosing & Administration
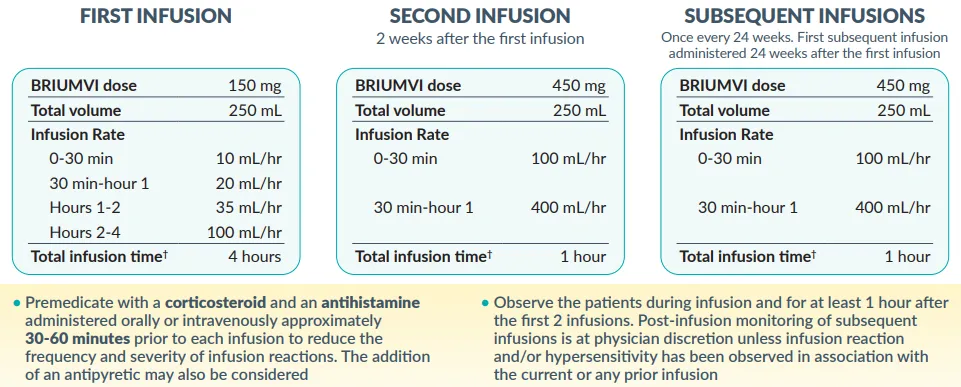
- First infusion: 150 mg IV over 4 hours
- Second infusion (2 weeks later): 450 mg IV over 1 hour
- Subsequent infusions (every 24 weeks): 450 mg IV over 1 hour.
- Premedication: Administer methylprednisolone (100 mg IV/oral equivalent) + antihistamine 30–60 minutes before infusion to mitigate reactions.
https://briumvihcp.com/wp-content/uploads/2024/10/BRIUMVI-Infusion-Rate-Card.pdf
Key Safety Considerations
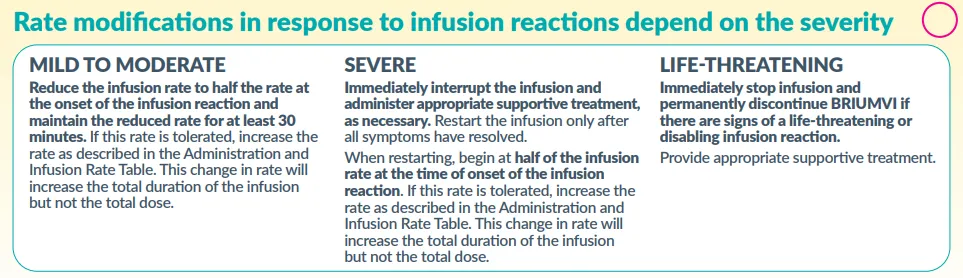
- Infusion-related reactions (IRRs):
- Occur in ~50% of patients (most common: headache, fever, chills).
- Management: Slow/interrupt infusion, restart at half-rate post-resolution.
- Infections & Immunosuppression:
- Screen for HBV (HBsAg, HBcAb) before initiation—contraindicated in active HBV.
- Avoid live vaccines ≥4 weeks pre-treatment and until B-cell repletion.
- Monitoring:
- Check serum immunoglobulins pre-treatment; monitor for hypogammaglobulinemia.
- Pregnancy testing recommended pre-infusion (FDA Category not yet assigned).
https://briumvihcp.com/wp-content/uploads/2024/10/BRIUMVI-Infusion-Rate-Card.pdf
Efficacy Highlights
- ULTIMATE I/II trials: Briumvi reduced annualized relapse rate (ARR) by 50% vs. teriflunomide (0.09 vs. 0.18).
- Fewer T1/T2 gadolinium-enhancing lesions vs. comparator.
Nursing & Patient Education
- Patient counseling: Emphasize infection vigilance, IRR symptoms, and vaccination timing.
- Infusion prep: Use aseptic technique; inspect for particulates/discoloration.
Briumvi’s shorter infusion time (vs. other anti-CD20 therapies) and biannual dosing may improve adherence and clinic workflow[1]. Ongoing monitoring for long-term safety (e.g., malignancy risk) is warranted.
References:
[1] https://pmc.ncbi.nlm.nih.gov/articles/PMC10553037/
[2] https://www.drugs.com/dosage/briumvi.html
[3] https://www.medicines.org.uk/emc/files/pil.100167.pdf
[4] http://impofy.com/importance-of-research-in-nursing/
[5] https://briumvihcp.com/study-design/
[6] https://briumvihcp.com/dosing-administration/
[7] https://www.drugs.com/briumvi.html
[8] https://quizlet.com/285368449/chapter-1-flash-cards/
[9] https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761238s000lbl.pdf
