Clinical and Pharmaceutical Differences Between Methylprednisolone Acetate and Methylprednisolone Sodium Succinate
Methylprednisolone acetate is a depot corticosteroid for prolonged anti-inflammatory effect via intramuscular or intra-articular injection. Sodium succinate is a water-soluble, rapid-onset formulation used intravenously for urgent systemic therapy
Introduction
Methylprednisolone is a synthetic corticosteroid widely used for its potent anti-inflammatory and immunosuppressive properties. Two common parenteral formulations used in clinical practice are methylprednisolone acetate and methylprednisolone sodium succinate. Although both contain the same active corticosteroid, these formulations are distinctly different in their chemical properties, pharmacokinetics, routes of administration, clinical indications, and preparation methods. Understanding these differences is essential to optimize therapeutic outcomes and minimize medication errors.
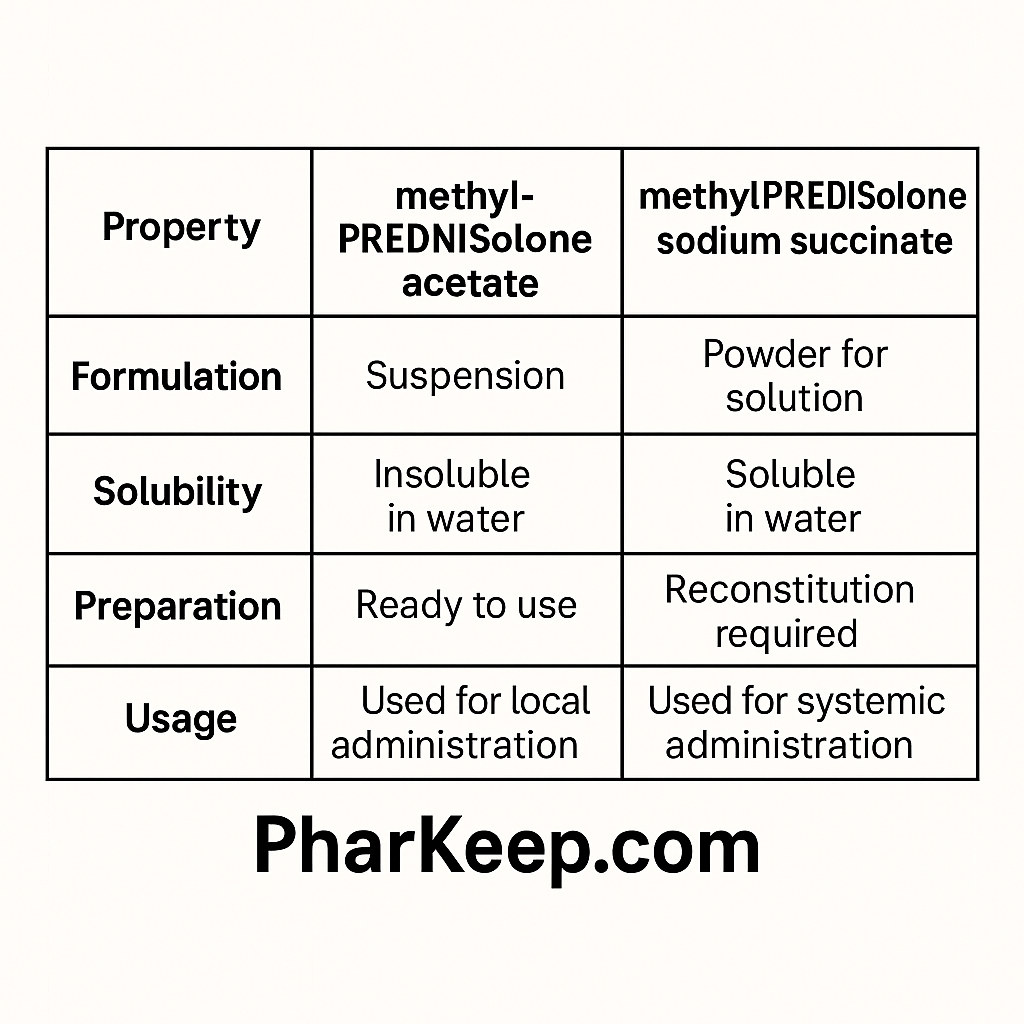
Chemical and Physical Characteristics
Methylprednisolone acetate is an almost insoluble white crystalline powder formulated as an aqueous suspension. This low water solubility is purposeful, designed to provide a depot effect with slow absorption over days to weeks. In contrast, methylprednisolone sodium succinate is a highly water-soluble sodium salt available as a sterile powder that is reconstituted with sterile diluent to form a clear solution suitable for intravenous or intramuscular injection.
Pharmacokinetics and Onset of Action
The pharmacokinetic profiles of these formulations reflect their physical properties. Sodium succinate, when injected intravenously, rapidly hydrolyzes to free methylprednisolone and exhibits a rapid onset of action—typically within one hour. It has a relatively short half-life with rapid clearance, suited for acute conditions needing immediate corticosteroid effects.
Methylprednisolone acetate’s insolubility results in slow and prolonged absorption from the injection site, providing sustained corticosteroid release over several weeks. This formulation is preferred when a long-lasting local effect is desired, such as for intra-articular or soft tissue injections.
Clinical Indications and Administration Routes
Methylprednisolone Sodium Succinate (Solu-Medrol®)
- Routes: Intravenous (IV), Intramuscular (IM)
- Indications: Emergency treatment of allergic reactions, acute exacerbations of asthma, multiple sclerosis relapses, shock, and high-dose pulse therapies.
- Preparation: Supplied as a sterile powder; reconstituted immediately before use. Requires slow IV administration to avoid cardiac adverse effects.
- Onset: Rapid (within 1 hour)
- Duration: Short; administration may be repeated every 4-6 hours if necessary.
Methylprednisolone Acetate (Depo-Medrol®)
- Routes: Intramuscular, intra-articular, intralesional, and soft tissue injection
- Indications: Osteoarthritis synovitis, rheumatoid arthritis, bursitis, tendonitis, and dermatologic conditions like keloids.
- Preparation: Ready-to-use aqueous suspension, must be vigorously shaken before administration; never given intravenously.
- Onset: Delayed (1 to several days)
- Duration: Prolonged effect lasting up to several weeks
Dosing and Administration Considerations
Methylprednisolone sodium succinate dosing varies widely based on indication, from 2 mg/kg in pediatrics to 30 mg/kg pulses in severe inflammatory states. It is critical to prepare this drug by proper reconstitution and adhere to IV administration guidelines to ensure efficacy and safety.
Methylprednisolone acetate dosing depends on the site and severity of inflammation, commonly 20-80 mg for large joint injections repeated every 1-5 weeks as needed. This depot formulation requires careful handling to avoid inadvertent intravenous administration.
Safety and Precautions
The formulations are not interchangeable. Inadvertent intravenous injection of methylprednisolone acetate suspension can cause embolism and severe complications. Knowledge of proper preparation, reconstitution, administration routes, and clinical indications is paramount to prevent medication errors. Sodium succinate formulations sometimes contain preservatives like benzyl alcohol, contraindicating use in neonates.
Summary
Methylprednisolone acetate and sodium succinate serve distinct roles in corticosteroid therapy based on solubility, pharmacokinetics, and clinical needs. Sodium succinate offers rapid systemic corticosteroid delivery crucial for emergencies, while acetate provides sustained local anti-inflammatory effects appropriate for musculoskeletal and dermatologic conditions. Mastery of the differences improves therapeutic outcomes and patient safety.
References
- FDA label: SOLU-MEDROL (methylprednisolone sodium succinate for injection) [PDF], U.S. FDA.
- Methylprednisolone Acetate - ScienceDirect Topics, Elsevier.
- FDA label: SOLU-MEDROL Prescribing Information, Pfizer.
- MedlinePlus Drug Information: Methylprednisolone Injection.
- FDA label: DEPO-MEDROL (methylprednisolone acetate injectable suspension), Pfizer.
- NCBI Bookshelf: Methylprednisolone - StatPearls.
- Methylprednisolone Acetate and Sodium Succinate overview - ScienceDirect.com.
- DrugBank: Methylprednisolone: Uses, Interactions, Mechanism of Action.
- Medscape Reference: methylprednisolone dosing and pharmacology.
- ISMP Canada safety bulletin: Depo-Medrol Confused with Solu-Medrol.
- Pharmacokinetics of methylprednisolone after intravenous and oral administration, PMC.
- Drugs.com: Depo-Medrol Dosage Guide.
- Mayo Clinic: Methylprednisolone Injection Uses & Side Effects.
- ISMP Canada: Medication error reports and safety tips.
- Clint Pharmaceuticals: Comparison of Corticosteroids.
- PubMed article: Pharmacokinetics of methylprednisolone, Wiley Online Library.
- Additional FDA-approved indications and preparation information from Pfizer labeling.
