New California Board of Pharmacy Compounding Rules: Expanded 2025 Update
California’s Board of Pharmacy has adopted comprehensive new compounding rules (June 2025). Updates cover sterile, non-sterile, and hazardous drug compounding, with expanded training, documentation, and facility requirements to protect patients and staff.
Overview
The Board of Pharmacy has overhauled Sections 1735–1738 of Title 16, California Code of Regulations, updating how pharmacies must compound medications. These changes bring California law into closer alignment with the U.S. Pharmacopeia (USP) standards:
- USP <795> – Nonsterile compounding
- USP <797> – Sterile compounding
- USP <800> – Hazardous drug handling
The goal: standardization, patient safety, and stronger oversight.
Expanded Key Updates
1. Alignment with Federal USP Standards
- Pharmacies must now follow USP <795>, <797>, and <800> in addition to California-specific rules.
- This ensures that state practices meet the same quality and safety standards used nationally.
- For sterile compounding, California specifically requires documentation of failures in cleanroom certification and mandates reporting to the Board within 72 hours.
2. Clearer Definitions and Scope
- Compounding vs. Reconstitution: Mixing outside FDA-approved directions counts as compounding.
- “Essentially a copy” of a commercial drug is restricted unless there is a drug shortage or a patient-specific clinical need is documented.
- Veterinary compounding: Allowed in limited quantities with restrictions on supply (14-day supply for nonsterile, 7-day for sterile, except ophthalmics).
3. Training and Competency
- Pharmacists and technicians must undergo initial and ongoing training in:
- Aseptic technique (for sterile compounding)
- Quality control and assurance
- Equipment handling and cleaning
- Failed competency evaluations mean staff cannot compound until retrained and revalidated.
- Facilities must document training and maintain competency records.
4. Personnel Hygiene and Protective Garb
- Staff with rashes, infections, or conditions that could contaminate drugs are prohibited from compounding areas.
- Disposable garb cannot be shared, must be replaced if soiled, and gloves must be changed between different compound components.
- For sterile compounding, piercings that risk contamination are specifically prohibited.
5. Facility and Environmental Standards
- Sinks used for handwashing may not be inside restrooms.
- Sterile compounding areas must maintain 20°C or cooler, with continuous or daily temperature monitoring.
- New rules for pass-through cabinets require interlocking doors.
- An existing secondary engineering control that has a pass-through that is not an interlocking device may continue to be used if the SOPs document that two doors may not be opened at the same time.
- Environmental monitoring (air and surface sampling) must follow CETA (Controlled Environment Testing Association) standards.
6. Documentation and Records
- Master Formulation Records (MFRs): Must include beyond-use date (BUD) justification, storage instructions, and references for stability data.
- Compounding Records (CRs): Must identify all components (manufacturer, lot, expiration), quantity compounded, staff involved, and verification steps.
- Audit Trail Requirement: All electronic and written records must allow tracking of revisions, with retention for 3 years.
7. Beyond-Use Dates (BUDs)
- Dates must be based on:
- Stability of active ingredients
- Container–closure compatibility
- Shortest expiration of any component
- If a BUD is listed only as a date, it expires at 11:59 PM on that day.
- Antimicrobial effectiveness testing must comply with USP <51>.
8. Release Testing and Quality Control
- Pharmacists remain responsible for compounded products until the BUD, assuming storage and handling instructions are followed.
- Injectable CSPs from nonsterile components must be tested for bacterial endotoxins per USP <85>.
- Any quality complaint must be investigated within 72 hours, and the Board notified within 96 hours.
9. Labeling Requirements
- Labels must include:
- Route of administration
- Special handling instructions
- Name and contact information of the compounding facility
- For infusions: the diluent, rate/duration, and instructions for use
10. Hazardous Drugs (USP <800>)
- Pharmacies must comply with federal standards for HD handling plus state-specific requirements.
- Covers: crushing, splitting, and repackaging antineoplastic agents.
- Requires documented facility lists of HDs, reviewed annually by the Pharmacist-in-Charge.
Why This Matters for Pharmacies
- Patient Safety: Reduces contamination, ensures potency, and prevents unsafe practices.
- Legal Compliance: Noncompliance could lead to fines, loss of licensure, or disciplinary action.
- Operational Impact: Pharmacies must update SOPs, train staff, and potentially upgrade facilities.
✅ Bottom Line:
By June 2025, all California pharmacies performing sterile, nonsterile, or hazardous drug compounding must fully comply with these updated regulations. This will require staff retraining, new documentation systems, and closer oversight by pharmacists-in-charge.
Click on image below to view side-by-side comparison chart (Old vs. New Rules)
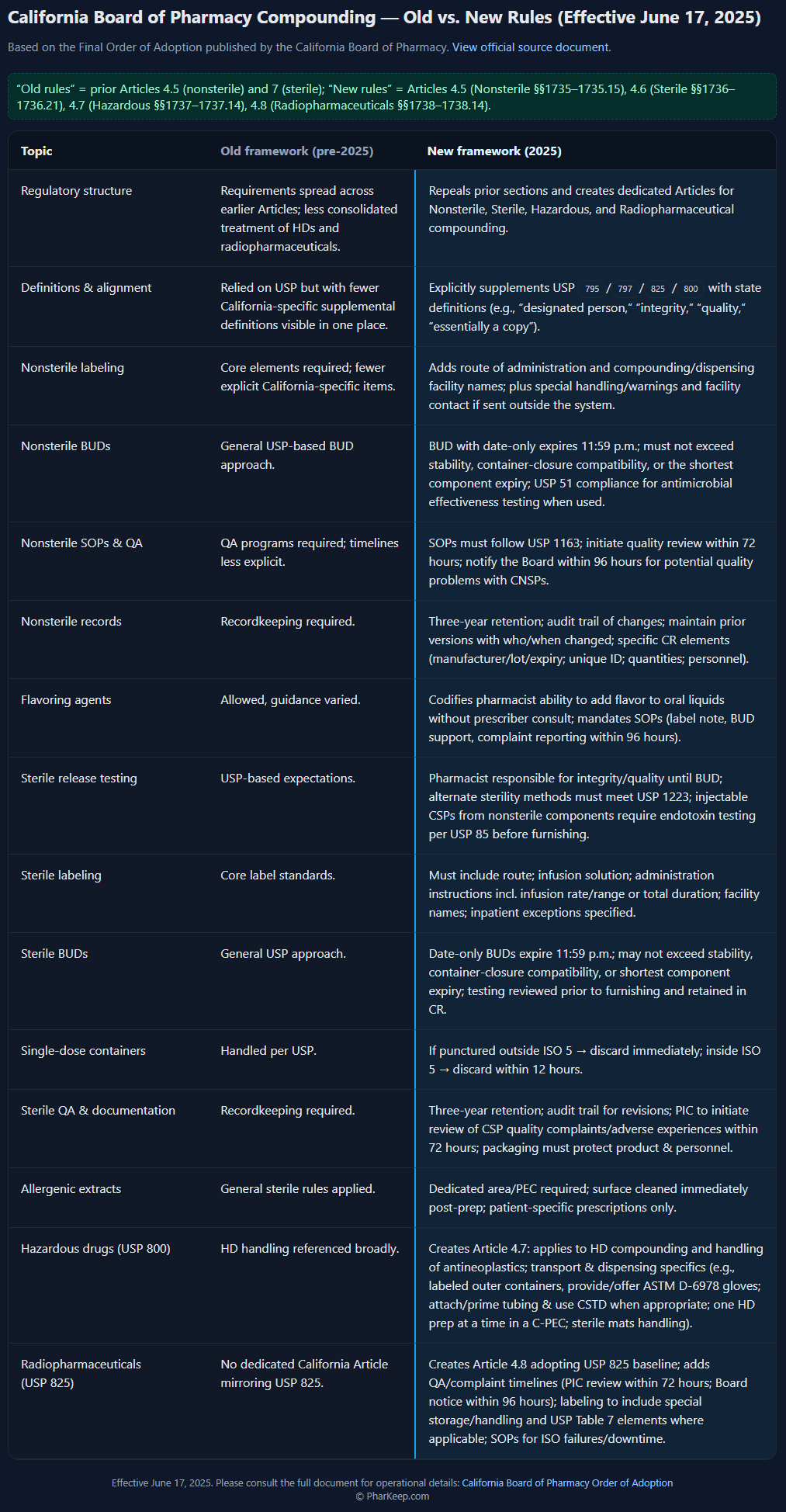
Based on the Final Order of Adoption published by the California Board of Pharmacy. View official source document.


